Press Release
Florham Park, NJ, May 1st, 2023
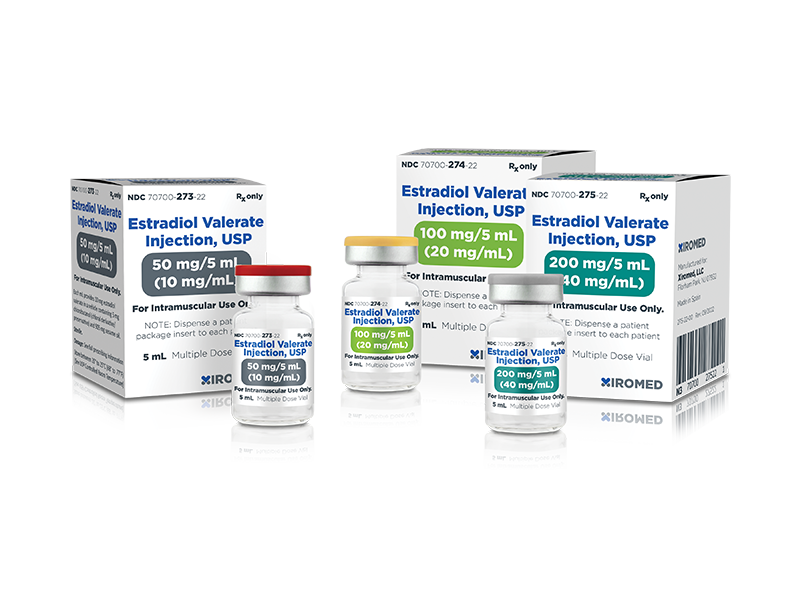
Xiromed LLC today announced that the U.S. Food and Drug Administration (FDA) has granted approval to its Abbreviated New Drug Application (ANDA) for Estradiol Valerate Injection, USP, generic to Delestrogen®. Xiromed’s Estradiol Valerate Injection, USP is available in 3 strengths: 10mg/mL (5mL), 20mg/mL (5mL) and 40mg/mL (5mL). All three strengths are available in a multiple dose vial. Xiromed will launch immediately.
Annual market sales for Delestrogen® and its generics for the twelve-month period ending March, 2023 were $29.8 million, according to IQVIA™.
Rob Spina, CEO of Xiromed commented, “Xiromed continues to execute on our strategy to bring hormonal injectable products to market in the US. Estradiol Valerate injection is Xiromed’s fourth product to launch from Laboratorios Farmalan, our dedicated hormonal injectable facility located in Leon, Spain. I am pleased to add another product to our women’s health generic portfolio, a core therapeutic area in Xiromed’s strategy to bring low-cost pharmaceutical product to US consumers.”
Xiromed LLC, located in Florham Park, NJ, is the US generic division of Insud Pharma, S.L., a global pharmaceutical group headquartered in Madrid, Spain. Xiromed is focused on developing and commercializing high quality generic pharmaceutical products for the US market. In addition to its commercial portfolio of generics available in the US, Xiromed has a robust development portfolio of generic pharmaceutical products in various stages of development, including injectable, inhalation and complex generic products. Learn more at http://www.xiromed.com/usa/