Florham Park, NJ, September 27, 2021
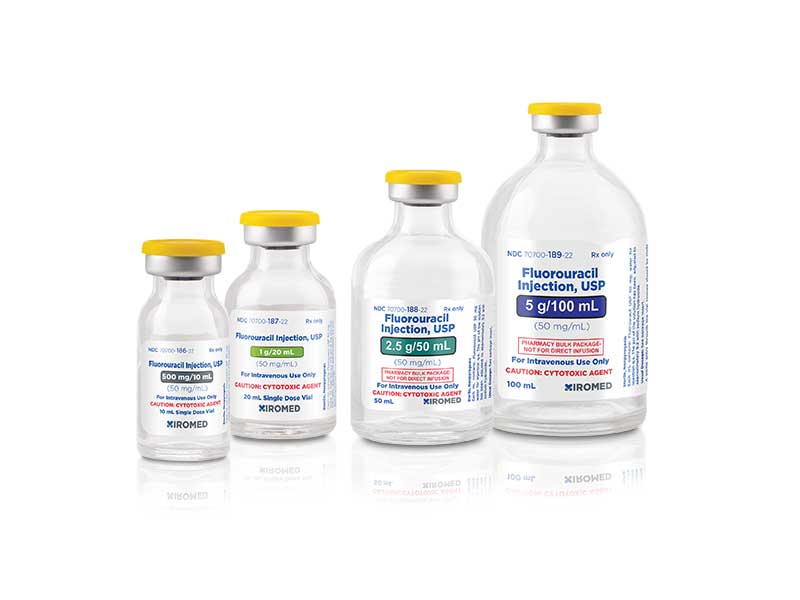
Xiromed LLC, the New Jersey-based generic division of Insud Pharmaceuticals, today announced the launch of Fluorouracil Injection 50mg/mL, generic to Adrucil®.
Annual market sales for Adrucil® and its generics for the twelve-month period ending June, 2021 were $22.2 million, according to IQVIA™.
Xiromed CEO, Narasimhan Mani commented, “The launch of Fluorouracil Injection 50mg/mL is the latest offering in our institutional portfolio as we continue to invest in a growing injectable pipeline to provide generic products to the US market.”
Xiromed LLC, located in Florham Park, NJ, is the US generic division of Insud Pharma, S.L., a global pharmaceutical group headquartered in Madrid, Spain. Xiromed is focused on developing and commercializing high quality generic pharmaceutical products for the US market. In addition to its commercial portfolio of generics available in the US, Xiromed has a robust development portfolio of generic pharmaceutical products in various stages of development, including injectable, inhalation and complex generic products. Learn more at http://www.xiromed.com/usa/
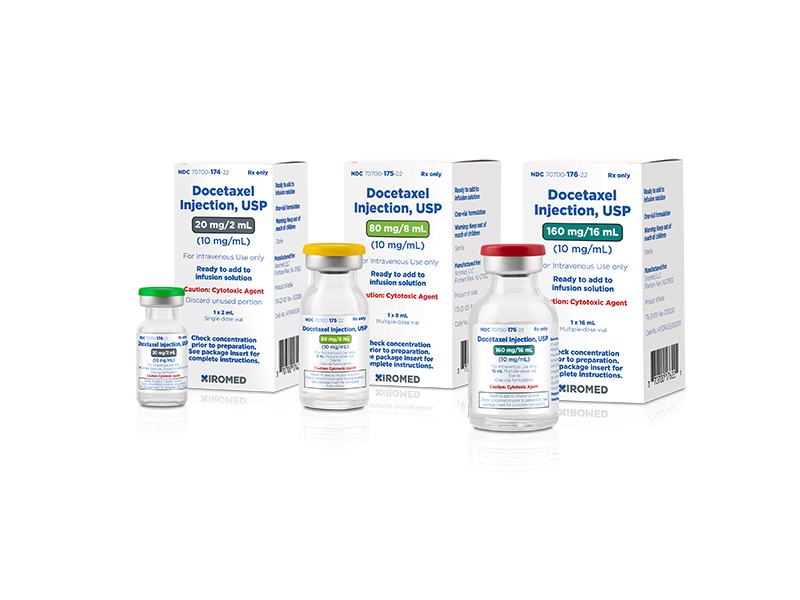
Xiromed LLC, the New Jersey-based generic division of Insud Pharmaceuticals, today announced the launch of Docetaxel Injection, USP, 10mg/mL generic to Taxotere®.
Annual market sales for Taxotere® and its generics for the twelve month period ending June, 2021 were $15.2 million, according to IQVIA™.
Xiromed CEO, Narasimhan Mani commented, “Xiromed continues to build a robust portfolio of generic injectable products for the US market. We are focused on providing value to our institutional customers with our injectable pipeline, and the launch of Docetaxel Vial for Injection is the latest example of that commitment.”
Xiromed LLC, located in Florham Park, NJ, is the US generic division of Insud Pharma, S.L., a global pharmaceutical group headquartered in Madrid, Spain. Xiromed is focused on developing and commercializing high quality generic pharmaceutical products for the US market. In addition to its commercial portfolio of generics available in the US, Xiromed has a robust development portfolio of generic pharmaceutical products in various stages of development, including injectable, inhalation and complex generic products. Learn more at http://www.xiromed.com/usa/