Glycopyrrolate Injection USP, 0.2mg/mL
GPI
25 x 1mL Single Dose Vial - 49-10-20-30-00-20-10 25 x 2mL Single Doses Vial - 49-10-20-30-00-20-12 25 x 5mL Multi Dose Vial - 49-10-20-30-00-20-13 10 x 20mL Multi Dose Vial - 49-10-20-30-00-20-14
GCN
19121
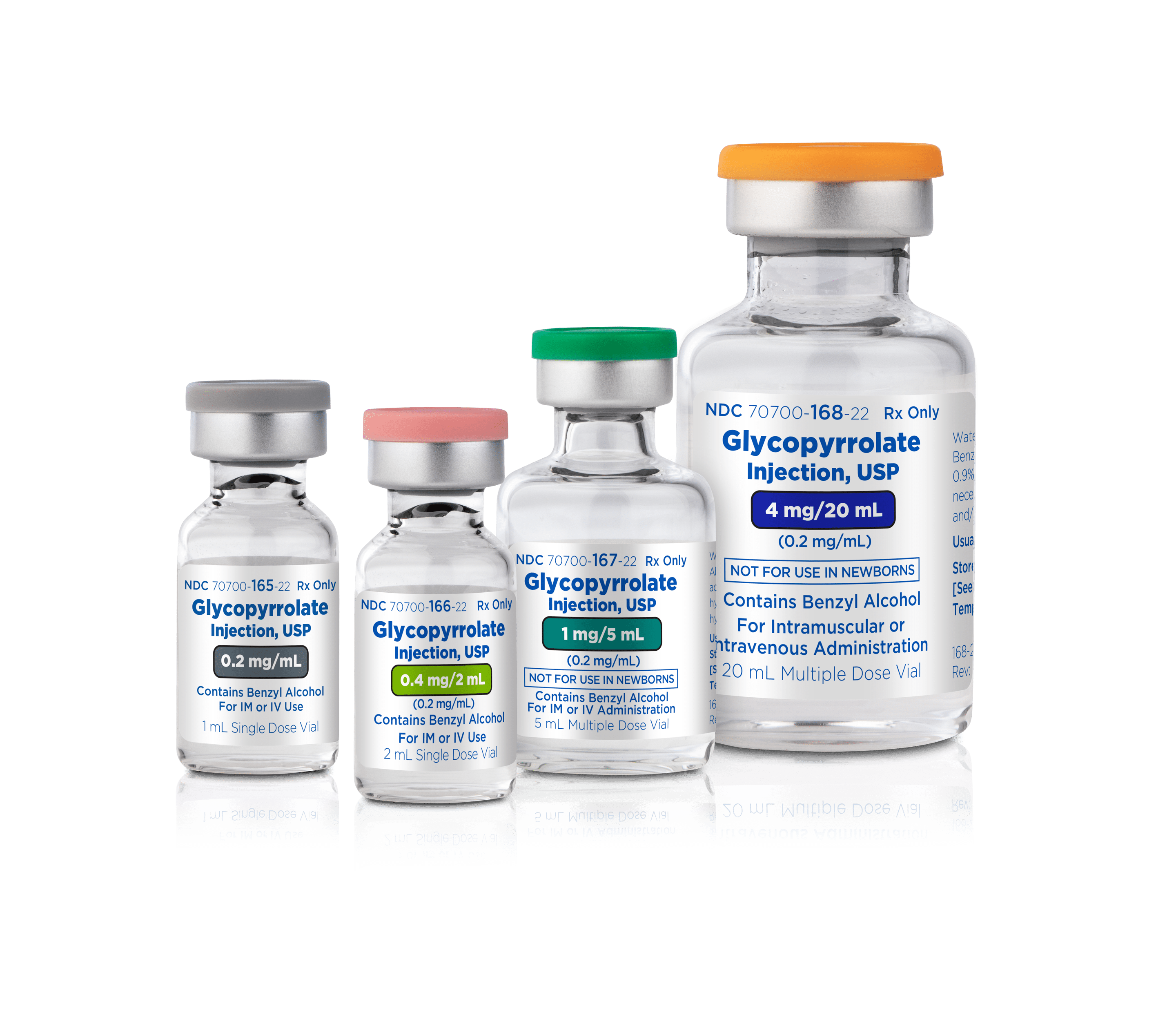
Available Sizes | NDC
25 x 1mL Single-Dose Vial|70700-165-25
25 x 2mL Single-Dose Vial|70700-166-25
25 x 5mL Multi-Dose Vial|70700-167-25
10 x 20mL Multi-Dose Vial|70700-168-23
Learn More about Glycopyrrolate Injection
Product Details
Product Name
Glycopyrrolate Injection USP, 0.2mg/mL
Strength
0.2mg/mL
0.4mg/2mL (0.2mg/mL)
1mg/5mL (0.2mg/mL)
4mg/20mL (0.2/mL)
Dosage Form
Injectable
Size
25 x 1mL Single-Dose Vial
25 x 2mL Single-Dose Vial
25 x 5mL Multi-Dose Vial
10 x 20mL Multi-Dose Vial
NDC
25 x 1mL Single-Dose Vial - 70700-165-25
25 x 2mL Single-Dose Vial - 70700-166-25
25 x 5mL Multi-Dose Vial - 70700-167-25
10 x 20mL Multi-Dose Vial - 70700-168-23
GCN
19121
GPI
25 x 1mL Single Dose Vial - 49-10-20-30-00-20-10
25 x 2mL Single Doses Vial - 49-10-20-30-00-20-12
25 x 5mL Multi Dose Vial - 49-10-20-30-00-20-13
10 x 20mL Multi Dose Vial - 49-10-20-30-00-20-14
UPC
25 x 1mL Single Dose Vial - 370700165256
25 x 2mL Single Doses Vial - 370700166253
25 x 5mL Multi Dose Vial - 370700167250
10 x 20mL Multi Dose Vial – 370700168233
OTC/Rx
Rx
TE Code
AP
Reference Brand
Robinul®
Status
Active
Case Qty (min. order qty)
25 x 1mL Single Dose Vial - 12
25 x 2mL Single Doses Vial - 12
25 x 5mL Multi Dose Vial - 12
10 x 20mL Multi Dose Vial – 1
Storage Conditions
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature]
Product Shelf Life
24 months
Shape
N/A
Color
Sterile clear, colorless solution
Imprint/Markings
N/A
Contains Gluten
The product does not contain gluten, or any ingredients derived from the gluten containing grains. However, the product is not certified as gluten free.
Contains Lactose
No
Latex
Container closure is not made with natural rubber latex
Contains Sweeteners
No
Contains Soy
The product does not contain soy, or any ingredients derived from soy. However, the product is not certified as soy free.
Contains Gelatin
No
Other Product Info
N/A
Note
N/A
Order Glycopyrrolate Injection Today!
Available in the following counts:
Available in the following strengths:
Pharmacology – Medical inquiry/complaint
If you are experiencing a medical emergency, please contact your health care provider. To speak with a medical information professional about your medical question, to report an adverse event or side effect, or concerns about the quality of a Xiromed product, call 844-XIROMED (844-947-6633) or contact us.

