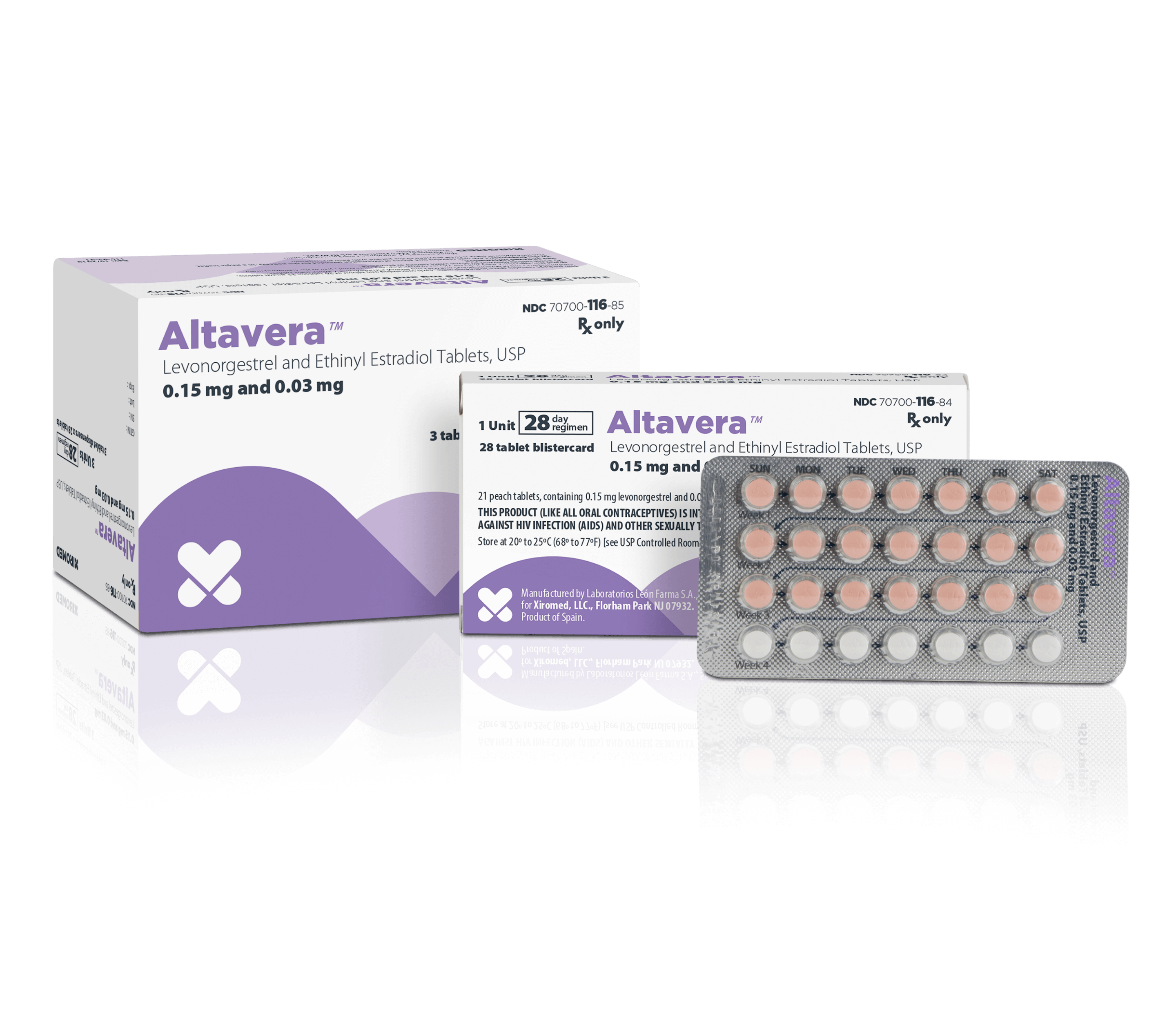
Altavera® (Levonorgestrel and Ethinyl Estradiol Tablets, USP)
GPI
25-99-00-02-40-03-10
GCN
11530
Available Sizes | NDC
3 x 28 Pack|70700-116-85
Learn More about Altavera®
Product Details
Product Name
Altavera® (Levonorgestrel and Ethinyl Estradiol Tablets, USP)
Strength
0.15 mg/0.03 mg
Dosage Form
Tablets
Size
3 x 28 Pack
NDC
70700-116-85
GCN
11530
GPI
25-99-00-02-40-03-10
UPC
370700116852
OTC/Rx
Rx
TE Code
AB
Reference Brand
Nordette-28®
Status
Active
Case Qty (min. order qty)
80
Storage Conditions
Store at 20° to 25°C (68° to 77°F).
Product Shelf Life
24 Months
Shape
Round
Color
Each blister card contains 21 peach active tablets and 7 inert white tablets
Imprint/Markings
The 21 active tablets are peach, round, film-coated, debossed with "SZ" on one side and "J4" on the other side. The 7 inert tablets are white, round, film-coated, debossed with "SZ" on one side and "J1" on the other side
Contains Gluten
The product does not contain gluten, or any ingredients derived from the gluten containing grains. However, the product is not certified as gluten free.
Contains Lactose
Yes
Latex
No
Contains Sweeteners
No
Contains Soy
The starting material used to make ethinyl estradiol may be derived from soy.
Contains Gelatin
No
Other Product Info
N/A
Note
Order Altavera® Today!
Available in the following counts:
Available in the following strengths:
Pharmacology – Medical inquiry/complaint
If you are experiencing a medical emergency, please contact your health care provider. To speak with a medical information professional about your medical question, to report an adverse event or side effect, or concerns about the quality of a Xiromed product, call 844-XIROMED (844-947-6633) or contact us.

